Abstract
Background: CD20 antibody plus alkylator and/or anthracycline based immunochemotherapy (IC) is a standard frontline therapy for patients with follicular lymphoma (FL) with 10-year event-free survival (EFS) and overall survival (OS) rates of approximately 50% and 80% respectively in long-term follow-up of clinical trials. Currently available clinical prognostic indices for FL have been designed using PFS and OS endpoints. Early events, commonly defined as progression of disease within 24 months (POD24) or early transformation to a more aggressive histology, are associated with inferior outcomes and increased risk of death due to refractory FL. Timely identification of the minority of patients with elevated mortality risk might enhance clinical management and research strategies. The FLIPI24 Consortium was created to develop a clinical prognostic index using early events as the primary endpoint. We report the outcomes for the pooled cohort and investigate the implications of therapy patterns on potential model development.
Methods: Individual patient data were pooled and harmonized from 11 prospective observational cohorts from Europe, North America, and Australia. Patients who were diagnosed with grades 1-3A FL and initiated frontline IC were eligible. EFS was defined as time from start of IC to progression, relapse, retreatment (2nd line), histologic transformation, or death due to any cause. Early events were defined using status at 24 months from start of IC. OS was defined as time from start of IC to death due to any cause. Kaplan Meier curves and Cox proportional hazards models were used to evaluate outcomes by clinical features and therapy types.
Results: 9006 patients were abstracted and harmonized, 6111 patients initiated frontline IC between 2002 and 2018 and were included in this analysis. Median age at diagnosis was 61 years (IQR 52-69) and 50% were male. Complete FLIPI data were available in 5637 patients (92%) and 46%, 32%, and 22% were low, intermediate, and high risk, respectively. IC type was 3079 R-CHOP or like (50%) , 1529 R-CVP or like (25%), 918 R-bendamustine (B-R) or like (15%), and 585 fludarabine or other alkylator based IC (10%); 3187 received CD20 antibody maintenance (52%). Patients receiving R-CHOP were younger, more frequently grade 3A, and more frequently had elevated LDH; differences in other characteristics by IC type were not clinically meaningful. At median follow-up of 42 months (IQR 17-72), 2647 patients (43%) had an event (any) and 1494 patients (25%) died. Median survival after an early (non-death) event was 49 months (95% CI: 41-58); 5-year OS was 46% (95% CI: 43-49) compared to 89% (95% CI: 88-90) in patients without POD24.
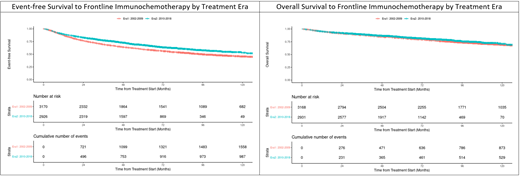
Across all IC types, EFS estimates at 2 and 10 years from start of IC were 80% (95% CI:79-81) and 49% (95% CI:48-51) and OS estimates were 92% (95% CI: 91-92) and 70% (95% CI: 69-72), respectively. FLIPI was highly associated with both EFS (c-statistic=0.61) and OS (c-statistic=0.65) from the initiation of IC (both p<0.0001). There were significant differences in EFS and OS by IC type (both p<0.0001) and use of maintenance was associated with prolonged EFS in landmark analyses at both 6 and 12 months from initiation of IC (both p<0.0001).
Treatment patterns changed significantly over the study timeframe. Use of B-R and/or maintenance increased to 30% and 70% respectively in N=2937 patients treated in 2010-2018 (Era2) compared to <1% and 40% respectively in N=3174 patients treated 2002-2009 (Era1). EFS was significantly higher for Era2 compared to Era1 (HR=0.77, 95% CI: 0.71-0.83), which remained significant after adjustment for FLIPI (EFS HR=0.82, 95% CI: 0.76-0.89). However, the association between treatment eras and overall survival was weaker (HR=0.89, 95% CI: 0.79-0.99) and not significant after adjusting for baseline FLIPI (OS HR=0.99, 95% CI: 0.88-1.10).
Conclusion: EFS and OS from this large pooled analysis of observational cohorts is similar to long-term follow-up of randomized clinical trials in the IC era and support the use of these data for model development. Modeling efforts for early events should adjust for initial IC selection and use of maintenance therapy. Utilization of bendamustine and/or maintenance therapy increased over the study timeframe from 2002-2018, and Era2 was associated with improved EFS but not OS. This cohort provides comprehensive and robust observational data to define clinical predictors in IC treated patients.
Maurer: Genentech: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Nanostring: Research Funding. Flowers: Janssen: Research Funding; Takeda: Research Funding; National Cancer Institute: Research Funding; Biopharma: Consultancy; BeiGene: Consultancy; Amgen: Research Funding; Celgene: Consultancy, Research Funding; Xencor: Research Funding; Acerta: Research Funding; Bayer: Consultancy, Research Funding; Sanofi: Research Funding; 4D: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; EMD: Research Funding; TG Therapeutics: Research Funding; Burroughs Wellcome Fund: Research Funding; Kite: Research Funding; AbbVie: Consultancy, Research Funding; Cellectis: Research Funding; Denovo: Consultancy; Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Epizyme, Inc.: Consultancy; Novartis: Research Funding; Nektar: Research Funding; Morphosys: Research Funding; Iovance: Research Funding; Spectrum: Consultancy; Pfizer: Research Funding; Ziopharm: Research Funding; Guardant: Research Funding; Eastern Cooperative Oncology Group: Research Funding; SeaGen: Consultancy; Pharmacyclics/Janssen: Consultancy; Genentech/Roche: Consultancy, Research Funding; Pharmacyclics: Research Funding. Villa: Janssen: Honoraria; Gilead: Honoraria; AstraZeneca: Honoraria; AbbVie: Honoraria; Seattle Genetics: Honoraria; Celgene: Honoraria; Lundbeck: Honoraria; Roche: Honoraria; NanoString Technologies: Honoraria. Weibull: Jansen-Cilag: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support. Ghesquieres: Janssen: Honoraria; Mundipharma: Consultancy, Honoraria; Roche: Consultancy; Celgene: Consultancy, Honoraria; Gilead Science: Consultancy, Honoraria. Kridel: Gilead Sciences: Research Funding. Gandhi: Janssen: Research Funding; Novartis: Honoraria. Cheah: Celgene: Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: advisory; Loxo/Lilly: Consultancy, Honoraria, Other: advisory; AstraZeneca: Consultancy, Honoraria, Other: advisory; AbbVie: Research Funding; Beigene: Consultancy, Honoraria, Other: advisory; Ascentage pharma: Consultancy, Honoraria, Other: advisory; Gilead: Consultancy, Honoraria, Other: advisory; MSD: Consultancy, Honoraria, Other: advisory, Research Funding; Janssen: Consultancy, Honoraria, Other: advisory; Roche: Consultancy, Honoraria, Other: advisory and travel expenses, Research Funding. Hawkes: Gilead: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees; Antigene: Membership on an entity's Board of Directors or advisory committees; Regeneron: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squib/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck KgA: Research Funding; Specialised Therapeutics: Consultancy; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accommodation expenses, Research Funding, Speakers Bureau; Janssen: Speakers Bureau. Seymour: Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sunesis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Mei Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Morphosys: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau. Freeman: Amgen: Honoraria; Celgene: Honoraria; Sanofi: Honoraria, Speakers Bureau; Incyte: Honoraria; Abbvie: Honoraria; Teva: Research Funding; Roche: Research Funding; Janssen: Honoraria, Speakers Bureau; Seattle Genetics: Honoraria; Bristol Myers Squibb: Honoraria, Speakers Bureau. Clausen: Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expences ASH 2019; Gilead: Consultancy, Other: Travel expences 15th ICML ; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wahlin: Gilead Sciences: Research Funding; Roche: Consultancy, Research Funding. Link: Novartis, Jannsen: Research Funding; Genentech/Roche: Consultancy, Research Funding; MEI: Consultancy. Ekstroem Smedby: Janssen Cilag: Research Funding; Takeda: Consultancy. Sehn: Genmab: Consultancy; Novartis: Consultancy; Debiopharm: Consultancy. Trněný: Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Portola: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Celgene: Consultancy; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria; Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. El-Galaly: ROCHE Ltd: Ended employment in the past 24 months; Abbvie: Other: Speakers fee. Cerhan: Celgene/BMS: Other: Connect Lymphoma Scientific Steering Committee, Research Funding; Regeneron Genetics Center: Other: Research Collaboration; Genentech: Research Funding; NanoString: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal